
Kongo Chemical co.,Ltd contributes to human healthcare as an innovative and skilled API-manufacturer.
Kongo Chemical co.,Ltd contributes to human healthcare as an innovative and skilled API-manufacturer.

『Establishment of quality 』
We always access to new synthesis technology, analysis technology, pharmaceutical affairs regulation, and we are working to research and development for "More better drug substance" to send to everyone. Based on accumulated organic synthesis system, search the most suitable synthesis root which is most suitable for industrialization and from design the goal product quality profile, our development of "More better drug substance" will start. Of course chemical properties, physical properties, cost, safety supply, the safe operation, provide information, delivery, speed are important, and having communication with custmer is improtant element for quality design. Our mission is to have a communicate and include required quality.
We promise the total support, the development of the GE and consignment pharmaceuticals.
Our R&D dept, has 3 groups which are "Process development G", "Development technology G", and "Analysis development G", and we use each technology and we create the process and products which ingratiate the customer's needs that "More better quality", "More safety" and "Cost reduction".
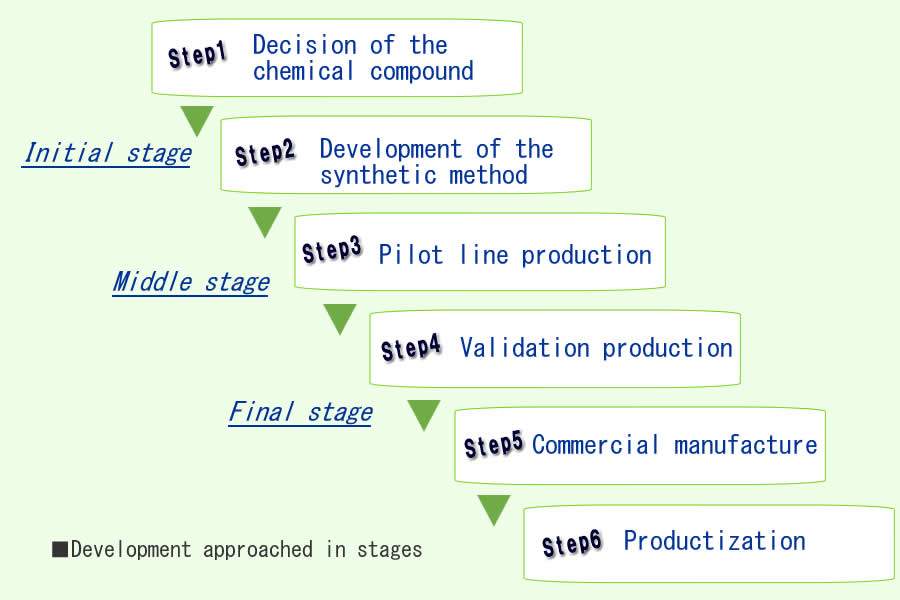
<Initial stage> Of course importance the speed and secure delivery, we suggest the improvement of based on development technology, new synthesis root and process, also suggest the problem of existing process. And we discuss about include the quality order of customer into which process. ■We work on development with the top priority in quality and deliverry, and brush up the development process, and suggest the safe development process. ■We create the necessary analysis method for the development process which suggested, and include the customer's quality needs which from initial stages of development.
<Middle stage to Final stage and Productization> ■From the laboratory and the trial data which were provided early in development, use the accumulated scale-up technology to guarantee the secure scale-up and process validation. ■For perform a reliable commercial production,we work together each sections, with efficient equipments and appropriate pharmaceutical affairs corresponding.
『 Maintenance of quality 』 We can build and share the development environment using all our resources. 「Quality Assurance Department」,「Manufacturing department」,「Sales department」,and more.
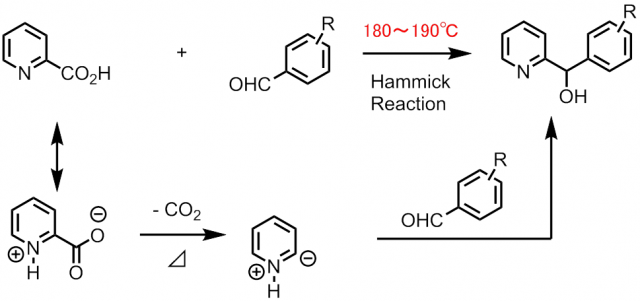
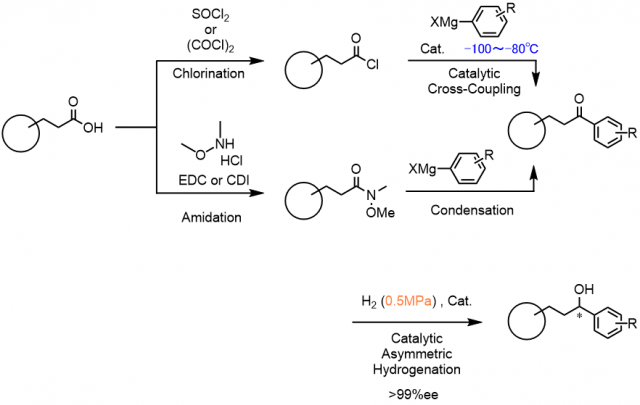
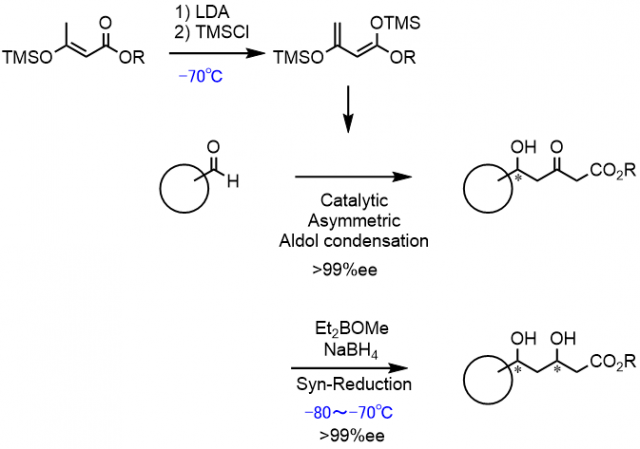
Since 1947,We are continuing the development of the technology.
<Manufacturing facility_example>
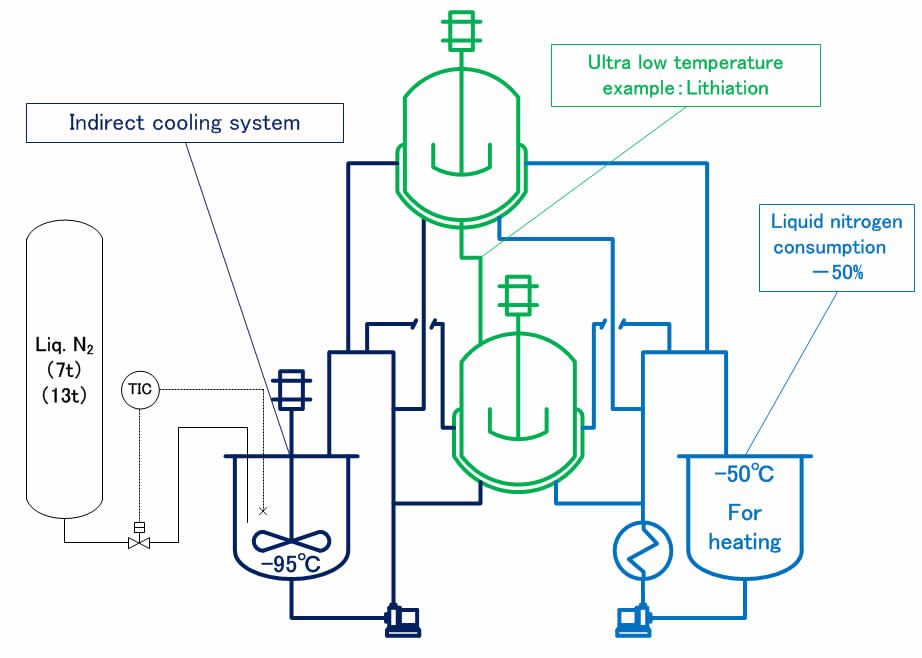
Brine indirect cooling system 『 Good soaking & temperature control 』 &『 Not brought into direct contact between reaction liquid and refrigerant 』&『 Robust system 』
Located in upper and lower /ultra-low temperature reaction tube 『 Can be dropped formulations in reaction liquid under the ultra low temperature 』
Selectively using the plurality of refrigerant 『 Reducing the manufacturing hours 』 &『 Energy reduction 』
| Small | 200L | SUS | located in upper and lower : 1 system |
|---|---|---|---|
| 400L | |||
| Medium | 1000L | SUS | located in upper and lower : 1 system |
| 2000L | |||
| Large | 4000L,6500L | SUS | located in upper and lower : 2 systems |
| 5200L,5200L |
<Manufacturing facility_example>
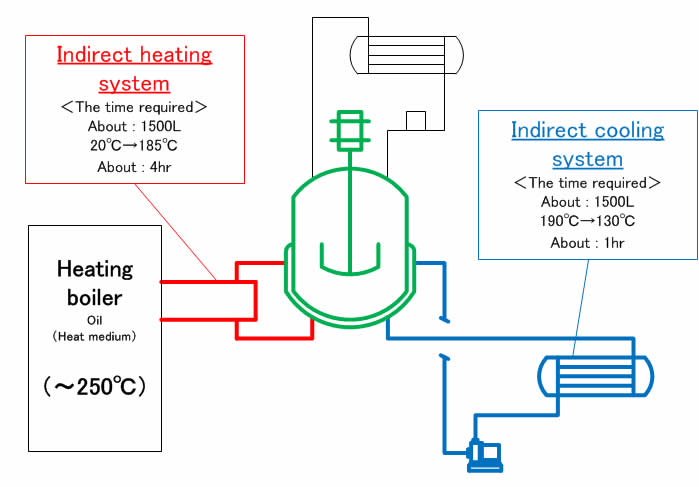
Indirect heating system/oil 『 Good soaking & temperature control 』 &『 Not brought into direct contact between reaction liquid and heat medium 』 &『 Robust system 』
High temperature specification GL Reactor 『 Adaptable various reaction conditions 』
Separate heating system from cooling system 『 Reducing the manufacturing hours 』 &『 Energy reduction 』
| Small | 200L | GL | up to 230℃ heat medium |
|---|---|---|---|
| Medium | 1000L | GL | up to 230℃ heat medium |
| 2000L | |||
| 3200L | up to 200℃ heat medium |
Details
・Amount to use/per year : 54,000㎥
・Catalyst : Pd-C,Raney-Ni etc.
・Respond to vapor‐liquid reaction : Scaling up of gas transfer coefficient
・Safety measures : Water leak detection method & Dispose hydrogen gas
| Small | 200L | GL | 1 |
|---|---|---|---|
| Medium | 1000L | SUS | 2 |
| 2000L | |||
| 2000L | GL | 1 | |
| Large | 4000L | GL | 1 |
| 5000L | 1 |